Master Polymer Plus Detection System (Peroxidase)
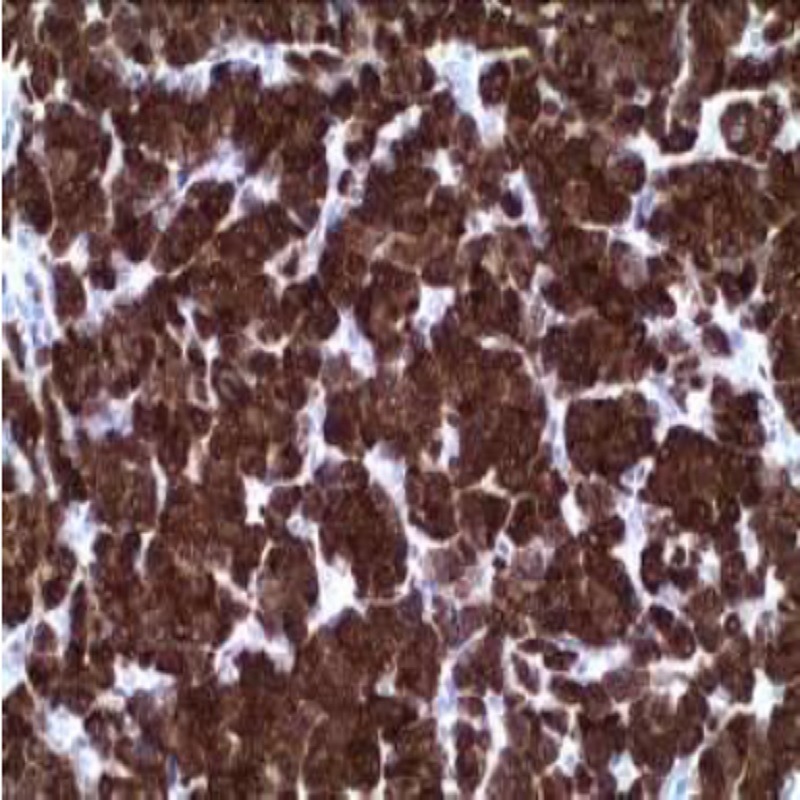
Application: This kit was developed for immunohistochemistry using monoclonal or polyclonal primary antibodies. After blocking the endogenous peroxidase and treating the tissues with the unlabeled primary antibody, the antigen-antibody complex y amplified by the primary antibody amplifier which is the detected by a complex component which consists in a micro-polymer marked with peroxidase
molecules. After predetermined times of incubation followed by abundant washings, the antigen+antibody+HRP polymer reaction is revealed with a DAB solution included in the kit.
Peroxidase Blocking Reagent (MAD-021540Q-10, MAD-021540Q-50 or MAD-021540Q-125): ready to use.
Primary Antibodies Amplifier Master (MAD-000237QK-B10, MAD-000237QK-B or MAD-000237QKB125):
signal amplifier compatible with mouse and rabbit primary antibodies. ; Ready to use.
Master Polymer Plus HRP (MAD-00237QK-C10, MAD-000237QK-C or Mad-000237QK-C125): micropolymer
constituted by the combination of conjugated secondary antibody and peroxidase
molecules, which recognizes mouse and rabbit primary antibodies; Ready to use.
DAB Substrate Buffer (MAD-001812QK-A, MAD-001811QK-A or MAD-001811QK-A125): Ready to use.
DAB Chromogen Concentrate (MAD-001812QK-B, MAD-001811QK-B or MAD-001811QK-B125):
Concentrated solution.
DAB enhancer (MAD-001560Q-10, MAD-001560Q-50 or MAD-001560Q-125): Ready for use.
Reagents and Accesory Material Required But Not Provided With The Kit:
1) Reagents: Primary antibodies, washing and HIER buffers, bidistilled water (distilled or deionized
water equivalent).
2) Accessories: IHQ microscopy slides and coverslips, pipettes, incubation moist chamber and
mounting medium.
Recommendations For Use:
TISSUE PREPARATION (for paraffin-embedded tissues)
The sample may undergo antigenic distortion if subjected to prolonged fixation and therefore for
maintaining its antigenic activity and an optimal bonding of the antibodies to the tissue, fixation with
10% buffered formalin for 24-48 hours is recommended.
SECTIONS PREPARATION (for paraffin-embedded tissues)
3-4μm thick sections should be taken on charges slides. For the first determinations it is advisable to
use positive and negative tissue samples or replace the primary antibody by washing buffer or
normal serum and processed them in the same way as the test sample.
STAINING PROCEDURE:
1. Blocking of endogenous peroxidase
– Apply 100ul of Peroxidase Blocking Reagent to each sample to completely cover the sections.
Incubate at room temperature for 10 minutes in darkness.
– Rinse slides in TBS three times for 5 minutes.
2. Primary antibody incubation
– Cover the tissue section with the adequate amount of solution, following the recommendations
given by the manufacturer.
– Rinse in TBS three times for 5 minutes.
3. Incubation with primary antibodies amplifier:
– Apply 100 μL of the Primary Antibodies Amplifier Master to each sample to completely cover the
sections. Incubate at room temperature for 15 minutes.
– Rinse in TBS three times for 5 minutes
4. Incubation with Master Polymer Plus HRP:
– Apply 100 μL of Master Polymer Plus HRP to each sample to completely cover the sections.
Incubate at room temperature for 30 minutes.
!!! Note: the micro-polymer solution is sensitive to light. Avoid unnecessary exposure to light and
store it in opaque vial or container.
– Rinse in TBS three times for 5 minutes.
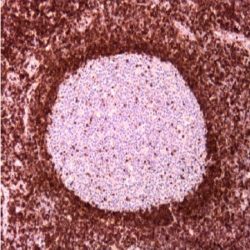
5. Immunostain visualization (Preparation and incubation with chromogen solution)
Chromogen solution preparation: Add 1 drop of DAB Chromogen Concentrate to 1 ml of DAB Substrate Buffer. Mix well. This solution should be protected from light. Under these conditions the
solution is stable for 24 hours.
– Apply the resulted chromogen solution to each sample to completely cover the sections.
– Incubate at room temperature for 5 minutes.
– Abundantly wash with distilled water 3 times for 5 minutes.
6. Staining intensifier (this step is not always required)
– Apply DAB Enhancer for 1-2 minutes at room temperature so as to cover the tissue samples.
– Rinse with distilled water 3 times for 5 minutes.
7. Counterstaining
– Cover the sample with hematoxylin for 1 min.
– Rinse well with distilled or deionized water.
8. Rinse and mount
– After abundantly washing with water, dehydration by successive steps of increasing alcohol
solutions, clear in xylene and mount with permanent mounting medium.



دیدگاهها
هیچ دیدگاهی برای این محصول نوشته نشده است.