Applicatin Of The Product: This Kit is developed for immunohistochemical study using monoclonal or polyclonal primary antibodies.
After incubating the selections with the unmarked primary antibody, the universal amplifier of primary antibodies is applied, followed by a complex constituted by micropolymer with universal secondary antibody and molecules of alkaline phosphatase. After the predetermined incubation times and after washing, it is developed using a specific chromogen of Alkaline Phosphatase (AP) type or the reagents of the developed kit.
Kit Components:
MAD-000230QK (500 test) MAD-000230QK-10 (100 test)
MAD-000230QK-B MAD-000230QK-B10 Primary Antibodies / Amplifier Master / AP
Primary antibodies / amplifier Master AP
MAD-000230QK-C MAD-000230QK-C10 Master Polymer / Plus AP
Polymer Master Plus / AP
MAD-001815QK-A MAD-001818QK-A AP Substrate Buffer / Substrate AP
MAD-001815QK-B MAD-001818QK-B AP Chromogen / Concentrate
Chromogen AP / concentrated / solution
Reagents and Accessories Needed Not Provided With The Kit:
1) Reagents: Reagents for the deparaffining and antigenic recovery for each antibody, primary antibodies,
washing buffers and bi-distilled water (distilled deionised water or equivalent).
2) Accessories: Microscope, slide and coverslip for microscopes, pipettes, sample tubes, humid hatchery
chamber and media mounting.
Recommendations For Use:
Sample Preparation (for paraffin-embedded tissues)
The sample can experiment an antigenic denaturalization if it is subjected to a prolonged fixation. Therefore,
and in order to obtain an optimal fixation with the tissue maintaining its antigenic activity, it is
recommended the fixation with 10% buffered formalin for 24-48 hours.
Sections Preparation (for paraffin-embedded tissues)
The sections are cut at 3μm and placed on the slides. If there is a need to do more treatments as antigenic
recovery, through heat or enzymatic treatment, the crystal slides must be covered with a sticker for tissue
sections as 0.02% poly-L-lysina or silane.
It is recommended to use a tissue sample with positive immunoreactivity and another one negative, or
substitute the primary antibody with washing buffer or normal serum and process them the same way as
the template sample for a correct interpretation of the staining results.
B. Staining Procedure:
1. Incubation with the primary antibody
– Covering the template tissue section following the recommendations provided by the manufacturer for the
use of this antibody.
– Clearing in TBS 3 times for 5 minutes.
2. Incubation with the amplifier of primary antibodies Master AP:
– Applying two drops (100 μL) of the Amplifier (Master Polymer Plus AP) to each one of the samples to
completely cover the sections. Incubating at room temperature for 10 minutes.
– Clearing in TBS 3 times for 5 minutes
3. Incubating with the Polymer Master Plus AP:
– Applying two drops (100 μL) of the micropolymer (Master Polymer Plus AP) to each one of the samples to
completely cover the sections. Incubating at room temperature for 15 minutes.
Note: The micropolymer is sensitive to the light. Avoid the unnecessary exposure to light and store in an
opaque vial or container.
– Clearing in TBS 3 times for 5 minutes.
4. Developing of the immunostaining (preparation and incubation with substrate/chromogen)
The mix substrate/chromogen must be preparedin the moment in which it is needed or, maximum, 30 minutes earlier.
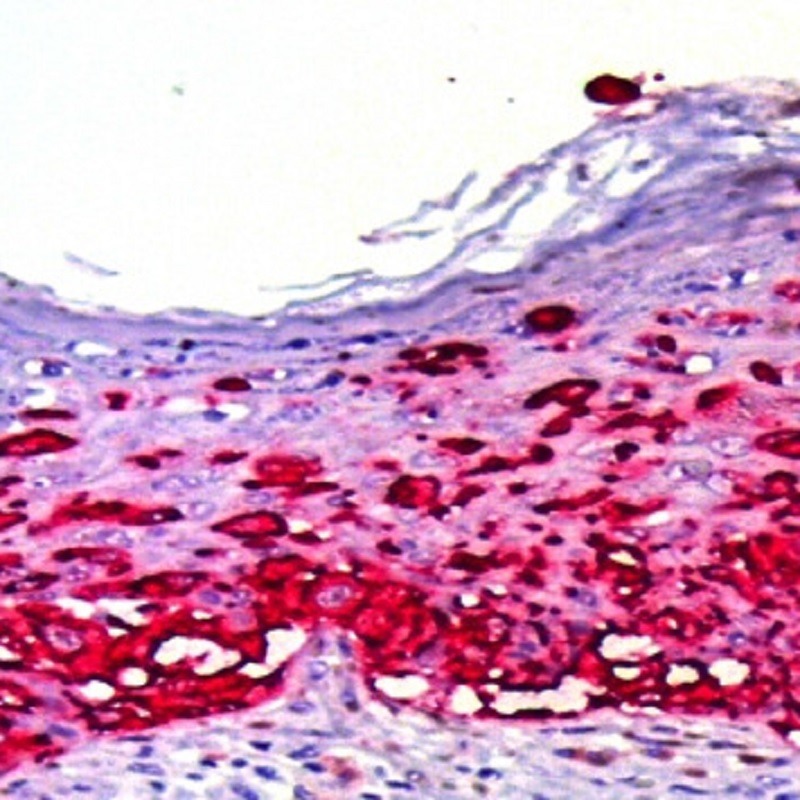
Adding 1 drop of red Chromogen AP concentrated at 2.5 ml of substrate buffer (or 2 drops at 5ml). Mix well. This solution must be safeguarded from light.
– Applying the mix substrate/chromogen to each one of the samples until completely covering the sections.
– Incubating at room temperature for 15 minutes.
– Washing with distilled water 3 times for minutes.
5. Sample contrast
– Covering the sample with haematoxylin for contract staining, for 1-2 min according to the intensity of the desired contrast.
– It is recommended not to use alcoholic solutions of contrast haematoxylin
– Washing properly with bi-distilled or deionized water. 6. Clearing and mounting
– After washing with water, drawing the slides at 50-60ºC for at least 30 minutes or 1 hour at room temperature, clearing in xylene and install with permanent mounting medium.

Reviews
There are no reviews yet.