Specification:
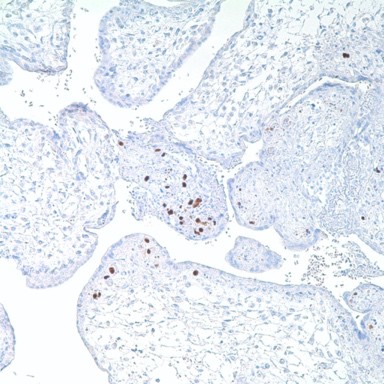
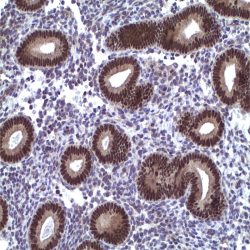
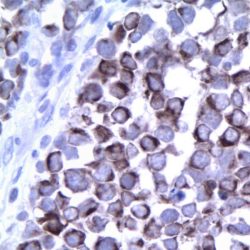
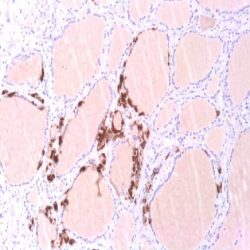
Human cytomegalovirus (CMV) is a β-herpesvirus (human herpesvirus 5) that causes widespread persistent infection. CMV continues to be an important opportunistic pathogen in immunocompromised patients. It is estimated that 30% of transplant recipients experience CMV disease.1 The range of organ involvement in post-transplant CMV disease is wide; hepatitis occurs in 40% of liver transplant recipients2, and pneumonitis is more frequently seen in heart and heart-lung transplant patients.3 Other organs that are commonly affected are the gastrointestinal tract and the peripheral and central nervous systems. Histologic diagnosis of CMV in fixed tissues usually rests on identifying characteristic cytopathic effects including intranuclear inclusions, cytoplasmic inclusions, or both, especially in the endothelial cells. However, histologic examination lacks sensitivity, and in some cases atypical cytopathic features can be confused with reactive or degenerative changes.4 Additionally, up to 38% of patients with gastrointestinal CMV disease fail to demonstrate any inclusions.5 In these cases, IHC using monoclonal antibodies against early and late CMV antigens allows the detection of CMV antigens in the nucleus and cytoplasm of infected cells. In addition, IHC may allow detection of CMV antigens early in the course of the disease when cytopathic changes have not yet developed.6 Various CMV antigens can serve as IHC targets for the detection of CMV infection. During productive, lytic CMV infection, the viral genes, encoding these antigens, are expressed in a coordinated and temporal fashion. The first viral genes expressed at 3-12 hours post-infection are the immediate early (IE) genes, which control viral and cellular gene expression to optimize the host environment for the production of viral DNA.7 IE gene expression is followed by viral early (E) gene transcription, or delayed early or intermediate early genes, usually expressed at 12-24 hours post infection. The E genes encode for proteins that are involved in viral DNA replication. Finally, E gene expression is followed by the transcription of viral late genes. The late genes encode for virus structural proteins.7 The sensitivity of IHC for detecting CMV infection ranges from 78% to 93%.4-5 The sensitivity of IHC is better than light microscopic identification of viral inclusions and compares favorably with culture and in situ hybridization.6-9 Additionally, immunohistochemical assays can be completed faster than the shell vial culture technique, allowing for rapid results that are important for early anti-CMV therapy.10 Immunohistochemistry has been used to detect CMV infection in patients with steroid refractory ulcerative colitis, and the routine use of IHC for the detection of CMV in the evaluation of these patients is now recommended.11-12 CMV immunostaining has been used to detect occult CMV infection of the central nervous system in liver transplant patients who develop neurologic complications.13 It has also been used to demonstrate a high frequency of CMV antigens in tissues from first trimester abortions.14
Presentation:
The product is formulated in a phosphate saline buffer (0.01M, pH 7.2) containing 0.1% sodium azide preservative

Reviews
There are no reviews yet.